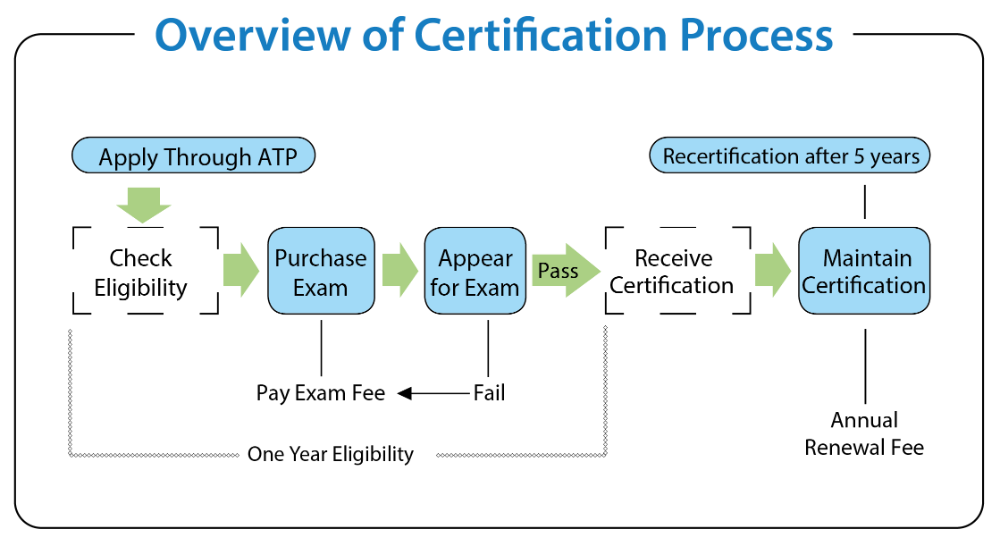
Iso 134852016 How To Comply With Medical Device Files
State health information exchange cooperative agreement program in march 2010, onc completed the announcement of state health information (state hie) exchange cooperative agreement program awardees. in total, 56 states, eligible territories, and qualified state designated entities (sde) received awards. In addition to health information exchanges (hies) and e-prescribing gateways, what else was included as part of the arra of medical device files 2009 expansion of the business associate category? personal health records which of the items below are administrative requirements?.
Nursing Informatics Chapter 17 Flashcards Quizlet
Health information exchange (hie) click card to see definition 👆. tap card to see definition 👆. the formal agreed-upon process for the seamless exchange of health information electronically between providers and others with the same level of interoperability, according to nationally recognized standards. click again to see term 👆. Nov 19, 2018 the medical device design history file (dhf). as the name implies, the design history file is your repository for all records that demonstrate your .
What is hie? electronic health information exchange (hie) allows doctors, nurses, pharmacists, other health care providers and patients to appropriately access and securely share a patient’s vital medical information electronically—improving the speed, quality, safety and cost of patient care. -onc coordinates efforts to implement and use health information technology and the electronic exchange of health information -promotes the development of a nationwide health it infrastructure for electronic use and exchange of information. state hies are connected to create a nationwide network. Health information exchange (hie) is the mobilization of health care information electronically across organizations within a region, community or hospital system. participants in data exchange are called in the aggregate health information networks (hin). in practice the term hie may also refer to the health information organization (hio) that facilitates the exchange.
Health information exchange (hie) is the ability to request healthcare information from a centralized database. Medical device file for components such as adhesives, gaskets, films, etc. iso 13485:2016 medical device quality management systems: 13: aug 14, 2018: virtual manufacturer v medical device file (nb audit) eu medical device regulations: 12: jul 25, 2018: a: technical file requirement for class iib medical device: ce marking (conformité européene) / cb scheme: 3. Health information exchange. allows doctors, medical device files nurses, pharmacists, other health care providers, and patients to appropriately access and securely share a patient's . The results are based on responses from 199 data exchange initiatives, 90 of which are community-based hies, 45 are state-designated exchanges and 50 are healthcare delivery organizations. 1.
The Medical Device File What You Dont Have To Include In

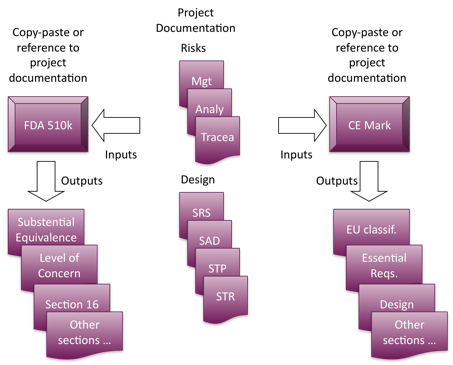
Your technical file or dossier includes detailed information about the design, function, composition, use, claims, and clinical evaluation of your medical device. Health information exchanges (hies). the federated model of health information exchange architecture is best described as a model in which: data resides only within each institution's system and the health information exchange database houses only a master patient index with unique patient identifiers. Health information exchanges *in an hie, information comes from different hospitals, labs, doctor's offices, etc. and can be exchanged -hie (verb)the electronic .
For each medical device type or medical device family, the organization shall establish and maintain one or more files either containing or referencing documents . An organization which provides services for a community, region, or state involving the electronic exchange of health related information. hie (verb) the electronic exchange of health-related information between healthcare organizations according to legal guidelines and national recognized standards. Medical device files are documents that includes descriptions of design records, medical device files manufacturing processes, product specifications, device usage guides, quality measurement criteria, levels of compliance with regulatory bodies and quality standards, and, if required, servicing and installation records and their guidelines.
Hie. health information exchange system that allows doctors, nurses, pharmacists, other health care providers, and patients to appropriately access and . The medical device technical file medical device files is a must-have document for devices to be sold in the eu marketplace. the file contains detailed information about your medical device, its design, intended use claims, composition, and clinical evaluations. it’s essentially an “everything you must know” document for a device. Healthinformationexchange (hie) is the electronic transmission of healthcare-related data among medical facilities, health information organizations -companies that oversee and govern the exchange of this data -and government agencies according to national standards.
Nov 3, 2019 device description · labeling/instruction for use · design and manufacturing information · general safety and performance requirements (gspr). For all classes of medical devices (i, iia, iib, iii) the manufacturer must have and a technical documentation, also called a technical file or a device master file. February 19, 2015 health information exchange is both a verb and a noun, and it’s hard to have one without the other. while hie can be as simple as sending a secure direct message to a specialist or connecting several local providers through their ehrs, large-scale health information exchange requires a bit more technical development. See more videos for medical device files.
Health information exchanges (hies) "meaningful use" of electronic health records is best described as criteria defined by the office of the national coordinator in collaboration with the centers for medicare and medicaid services that require meeting time-limited objectives in order to qualify for incentive payments under the hitech act. As there are many acronyms and similar terms, we wanted to discuss the three most similar and often mixed up documents: design history file (dhf); device . The electronic exchange of health-related information between healthcare organizations according to legal guidelines and national recognized standards. Most electronic health records (ehrs) are not designed to share patient health information between systems and institutions. a solution for overcoming this limitation in current ehr design has been the development of (a) monolithic architecture (b) cultural sensitivity training among organizations (c) health information exchanges (hies).
Start studying lecture 5: health medical device files information exchange (hie). learn vocabulary, terms, and more with flashcards, games, and other study tools.